ACUTE LIMB ISCHEMIA.
REAL WORLD. REAL PATIENTS.
REAL DIFFERENCES.
PROWL Registry: Outcomes using Pounce™ Mechanical Thrombectomy

ACUTE LIMB
ISCHEMIA.
REAL WORLD.
REAL PATIENTS.
REAL DIFFERENCES.
PROWL Registry: Outcomes using Pounce™ Mechanical Thrombectomy

What is the PROWL Registry?
Real-world study in adult patients presenting with symptoms of acute, subacute, or chronic limb ischemia in whom the Pounce™ Thrombectomy System was used for thromboemboli extraction.
Procedural Success: Restoration of pulsatile flow in the target lesion(s) with or without adjunctive treatment (Patient-level success)
Technical Success: Restoration of blood flow to the target lesions(s) with <50% residual obstruction without the need to initiate CDT or to proceed to open surgery or other endovascular thrombectomy device (Lesion-level success)
Study Cohort
Real-World, Multicenter
Core-Lab
Adjudicated
≤ 500 Patients
Up to 30
Sites
30-days Post
Procedure
All Comers
Why is the PROWL Registry unique?
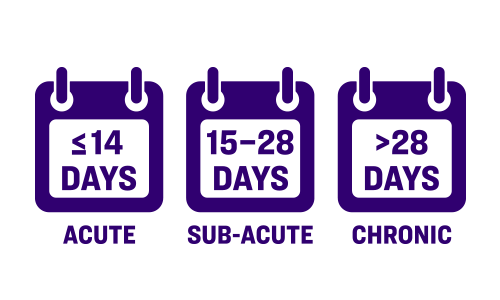
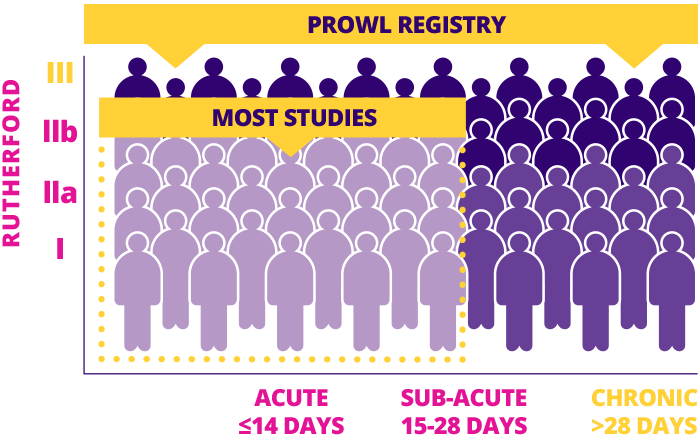
One of the only studies to include ALL THROMBOTIC clinical presentations and Rutherford scores
MECHANICAL (NON-ASPIRATION) THROMBECTOMY
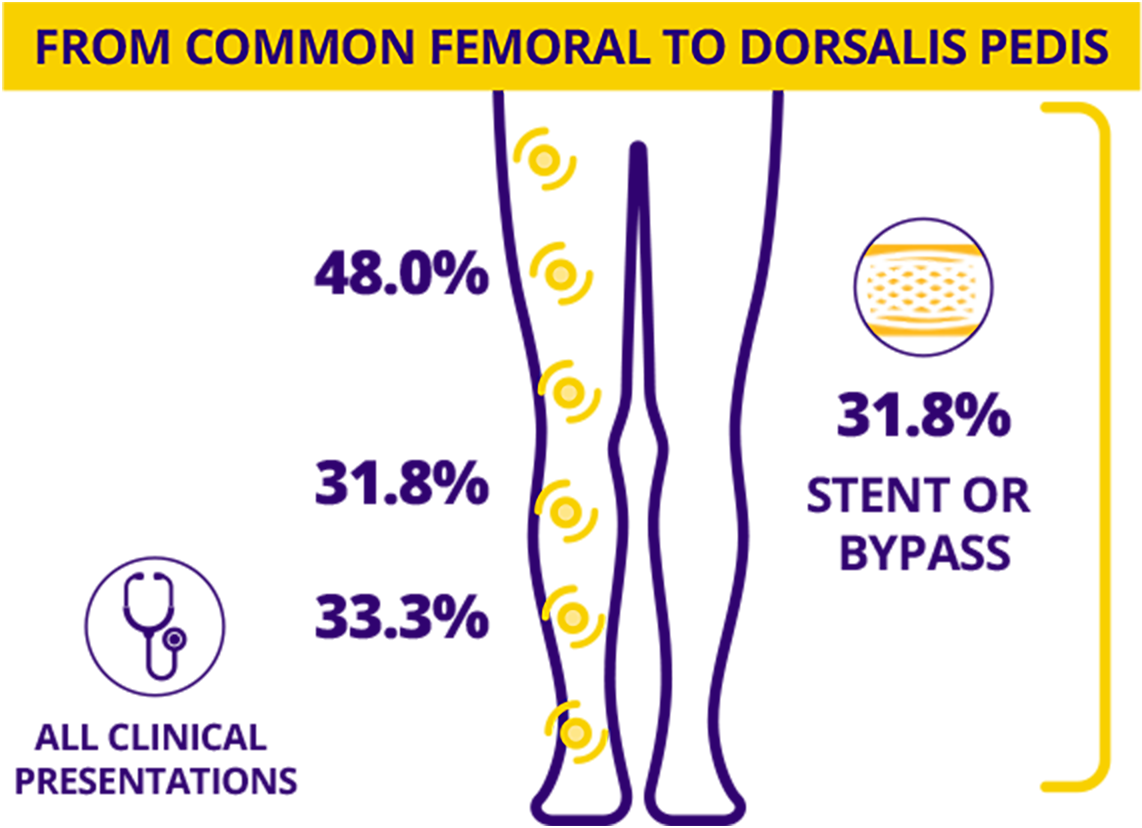
All Major Leg Vessels
– All Types
Vessel segment(s) treated are not mutually exclusive.
The Pounce™ Thrombectomy Platform allowed for significant efficacy with high procedural success.1
SAFETY
2.0 24.1min
Median Number of Passes per Patient
N = 160 Patients
Device
Time
SUCCESS
91.7%
Procedure
Success
9 Sites
SAFETY
ZERO 0.6%
Device-Related Embolizations*
(1 in 160)
Device-Related
CD-TLR
SAVINGS
64.4%
Avoided
ICU Time
DATA UNLEASHED AS OF 11/03/25
New Findings: Decide with Data
- Lyden S. Real-world Clinical Outcomes and Case Insights of the Novel Pounce™ Thrombectomy Platform. 23rd Annual Vascular InterVentional Advances (VIVA); November 3rd, 2025; Las Vegas, Nevada.
- Distal embolization requiring surgical procedure or obstructing one of the major downstream vessels >70% (at the end of the procedure).
DATA UNLEASHED
AS OF 11/03/25
New Findings:
Decide with Data
N = 160 Patients
9 Sites
The Pounce™ Thrombectomy Platform allowed for significant efficacy with high procedural success.1
SPEED
2.0 24.1min
Median Number of Passes per Patient
Device
Time
SUCCESS
91.7%
Procedure Success
SAFETY
ZERO 0.6%
Device-Related Embolizations*
(1 in 160)
Device-Related
CD-TLR
SAVINGS
64.4%
Avoided ICU Time
- Lyden S. Real-world Clinical Outcomes and Case Insights of the Novel Pounce™ Thrombectomy Platform. 23rd Annual Vascular InterVentional Advances (VIVA); November 3rd, 2025; Las Vegas, Nevada.
- Distal embolization requiring surgical procedure or obstructing one of the major downstream vessels >70% (at the end of the procedure).

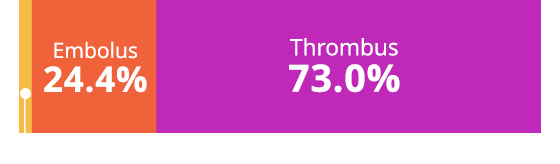
Clot Type
Embolus
24.4%
Thrombus
73.0%
Unknown/Not Reported 2.7%
Clinical Presentation
88.6%
of ALI subjects had immediately threatened limbs
30.0%
of subjects had chronic
symptoms for 3.4 months average (ranges up to 12 months)
Symptom Onset
Data on up to 500 patients (all comers) at up to 30
sites through 30 days
post-procedure
160 infrainguinal patients
reported here
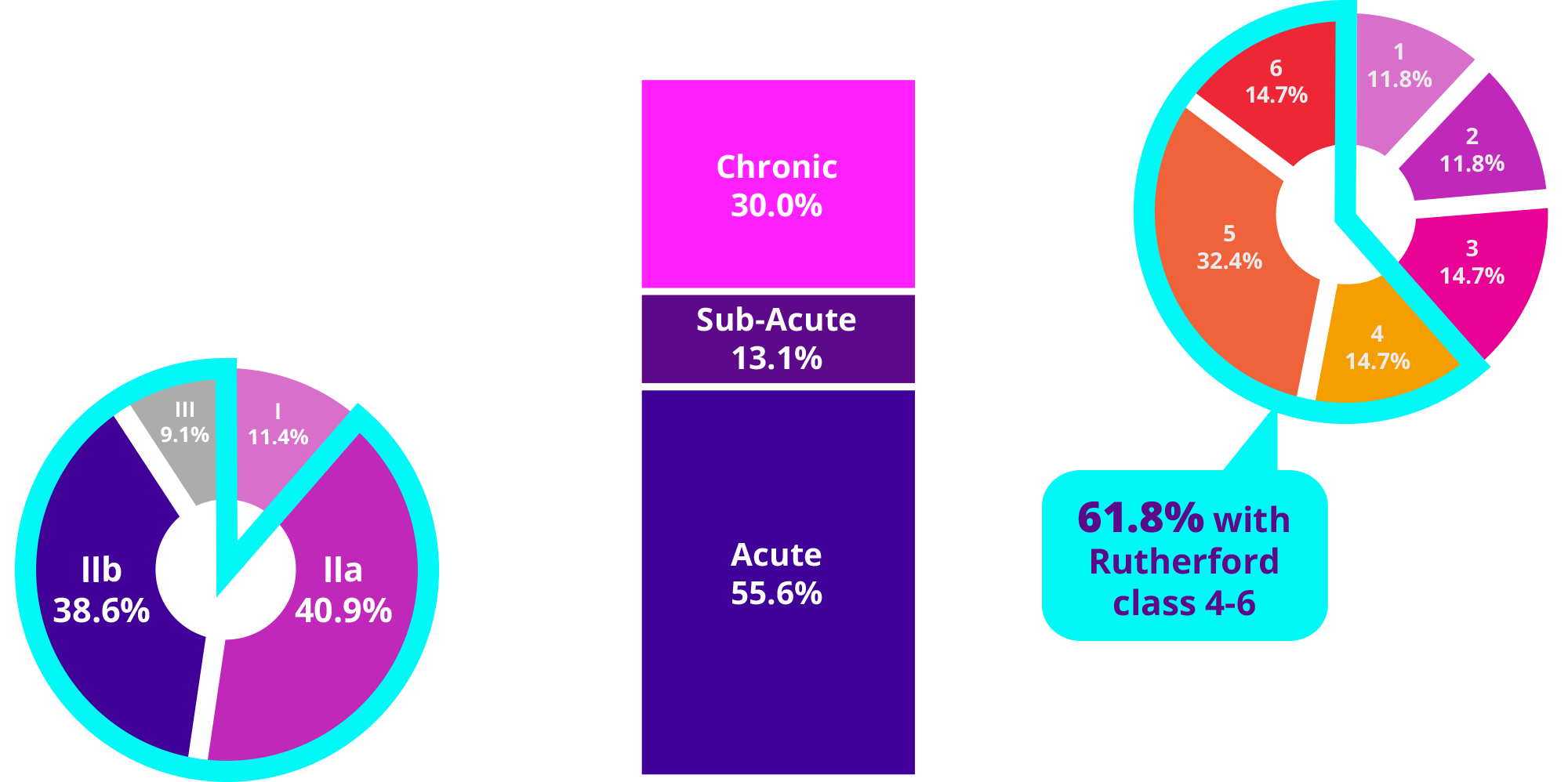
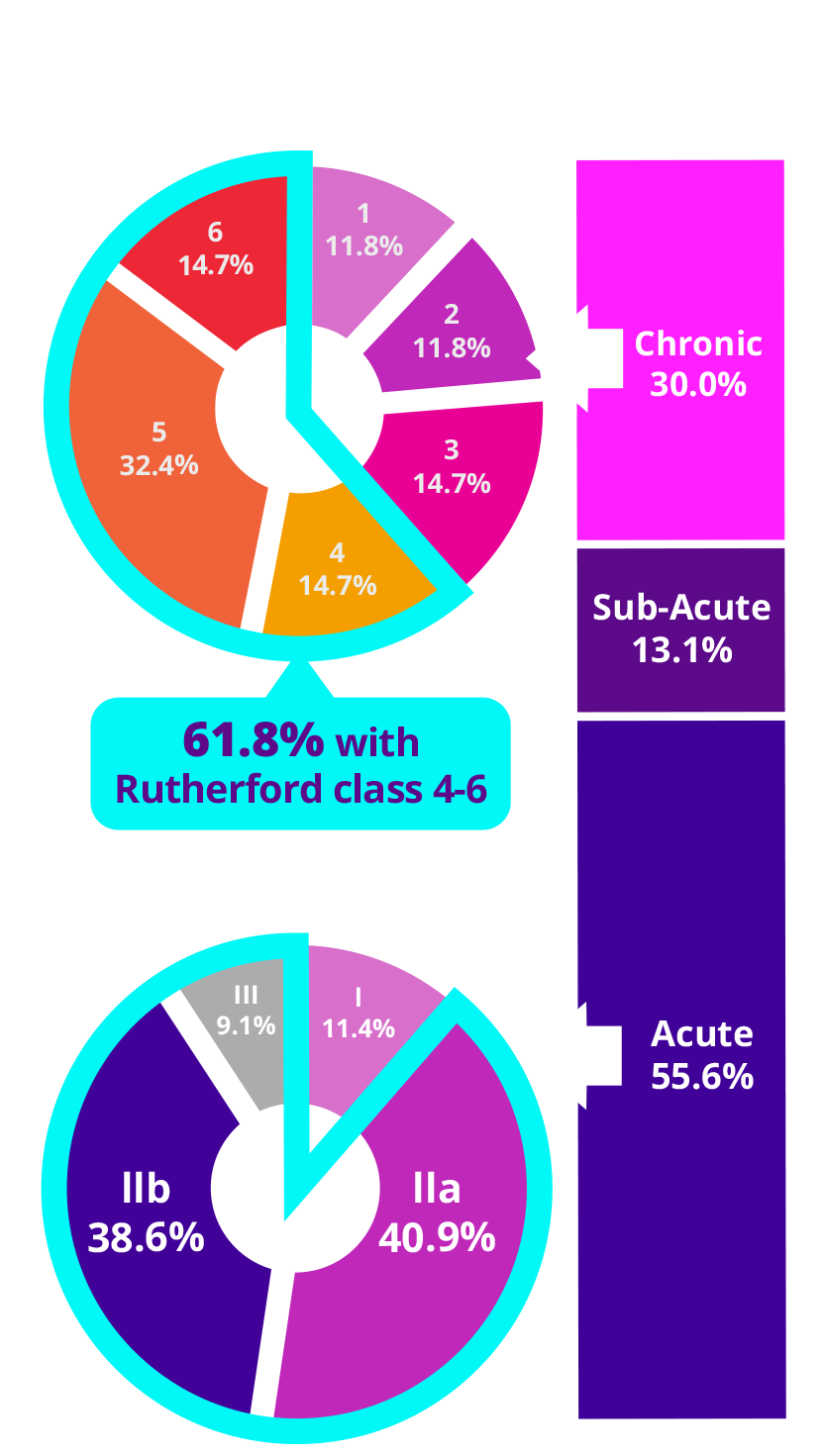
CLINICAL PRESENTATION
TOTAL SUBJECTS (N=160)
>60.0%
of sub-acute and chronic thrombotic subjects had Rutherford classification 4-6
Chronic Rutherford
Acute Rutherford

Clot Type
Symptom Onset
Clinical Presentation
Data on up to 500 patients (all comers) at up to 30 sites through
30 days post-procedure
160 infrainguinal patients reported here
88.6%
of ALI subjects had immediately threatened limbs
30.0%
of subjects had chronic symptoms for 3.4 months average (ranges up to 12 months)
>60.0%
of sub-acute and chronic thrombotic subjects had Rutherford classification 4-6
- Key procedure adverse events
- Device related flow limiting dissection: 0.6%
- No device-related significant embolizationA
- No device-related major bleeding requiring transfusion
- 30-day all-cause major adverse events
- Major amputation: 8.1%
- CD-TLR: 7.5%
- Mortality: 4.4%B
EFFICACY ENDPOINTS
- Technical Success: 83.2% of target lesions
- Restoration of blood flow to the target lesions(s) with <50% residual obstruction without the need to initiate CDT or to proceed to open surgery or other endovascular thrombectomy device.
- Procedural Success: 91.7% of subjects
- Restoration of pulsatile flow in the target lesion(s) with or without adjunctive treatment
- Complete or Substantial Thrombus Removal (Core lab):
- 94.1% at end of procedure
- 76.1% Following use of Pounce platform
- Arterial flow (Core Lab): 94.8% TIPI Grade 2/3
SAFETY ENDPOINTS
- Requiring surgical procedure or obstructing one of the major downstream vessels >70% (at the end of the procedure)
- Reason for death: 2 AKI, 1 Shock, 1 Sepsis, 1 Organ Malperfusion, 2 Unknown; none device related
Stay up-to-date with the latest PROWL Registry data
Intended Use:
The Pounce™ Thrombectomy System is intended for the non-surgical removal of thrombi and emboli from the peripheral arterial vasculature.
Contraindications:
The device is contraindicated for use in patients who cannot receive recommended intravenous anticoagulant therapy.